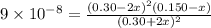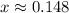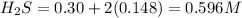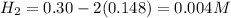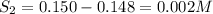For the equilibrium
2H2S(g) ⇋ 2H2(g) + S2(g) Kc = 9 .0X 10-8 at 700°C
the initial concentrati...

Chemistry, 06.07.2021 23:10 loganrose50
For the equilibrium
2H2S(g) ⇋ 2H2(g) + S2(g) Kc = 9 .0X 10-8 at 700°C
the initial concentrations of the three gases are 0.300 M H2S, 0.300 M H2, and 0. 1 50 M S2' Determine the equilibrium concentrations of the gases.

Answers: 2


Another question on Chemistry




Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1
You know the right answer?
Questions


Biology, 24.01.2021 23:10

Mathematics, 24.01.2021 23:10



History, 24.01.2021 23:10


Mathematics, 24.01.2021 23:10



Mathematics, 24.01.2021 23:10








Biology, 24.01.2021 23:20

Mathematics, 24.01.2021 23:20

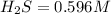
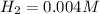
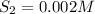
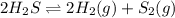
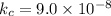
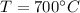
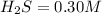
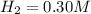
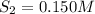
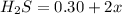
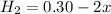
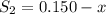
![K_c=\frac{product}{Reactant}=\frac{[H_2]^2[S_2]}{[H_2S]^2}](/tpl/images/1390/0282/9b893.png)