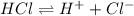Chemistry, 07.07.2021 01:00 laytonlutz
A Bronsted-Lowry acid is defined as a substance that . A Bronsted-Lowry acid is defined as a substance that . increases Ka when placed in H2O increases [OH-] when placed in H2O acts as a proton donor acts as a proton acceptor decreases [H ] when placed in H2O

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3
You know the right answer?
A Bronsted-Lowry acid is defined as a substance that . A Bronsted-Lowry acid is defined as a substan...
Questions

Computers and Technology, 24.04.2020 19:54










Spanish, 24.04.2020 19:54

Mathematics, 24.04.2020 19:54


Spanish, 24.04.2020 19:54




Mathematics, 24.04.2020 19:54