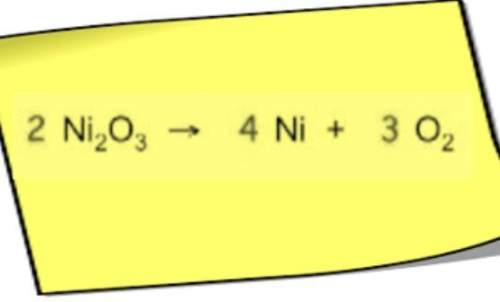
Chemistry, 07.07.2021 18:20 crystalclear99
Increasing the temperature of a chemical reaction usually increases greatly the rate of the reaction. The most iportant reason for this is that increasing the temperature increases: .
A) the collision frequency
B) the probability factor
C) the fraction of collisions with energy greater than Bact
D) the energy of activation.
E) the amount of heat released in the reaction

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 03:00
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3

You know the right answer?
Increasing the temperature of a chemical reaction usually increases greatly the rate of the reaction...
Questions



Mathematics, 03.11.2020 15:00




Mathematics, 03.11.2020 15:00

Biology, 03.11.2020 15:00



Arts, 03.11.2020 15:00


Geography, 03.11.2020 15:00




History, 03.11.2020 15:00

English, 03.11.2020 15:00