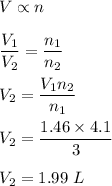Chemistry, 08.07.2021 04:40 homeschool0123
A reaction produces 3.0 mol of gas, which occupies 1.46 L. What is the volume of the product when 4.1 mol are produced at constant temperature and pressure?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3

Chemistry, 22.06.2019 16:00
Which process transfers heat from inside earth to its surface? convection currents in mantle pulling away of tectonic plates drawing in of tectonic plates convection currents in crust
Answers: 1

Chemistry, 23.06.2019 01:00
Heat energy, carbon dioxide, and water are released through which process? a. photosynthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1

Chemistry, 23.06.2019 08:00
Pl what kind of reaction is this? nahco3 + h2o → co2 + naoh + h2o -composition -decomposition -single replacement -double replacement im leaning more toward single replacement. if im wrong can you explain whyy?
Answers: 2
You know the right answer?
A reaction produces 3.0 mol of gas, which occupies 1.46 L. What is the volume of the product when 4....
Questions


Mathematics, 17.05.2021 18:30




Mathematics, 17.05.2021 18:30

English, 17.05.2021 18:30


History, 17.05.2021 18:30

Biology, 17.05.2021 18:30


Chemistry, 17.05.2021 18:30

Computers and Technology, 17.05.2021 18:30


Mathematics, 17.05.2021 18:30


Physics, 17.05.2021 18:30


English, 17.05.2021 18:30

Mathematics, 17.05.2021 18:30