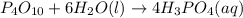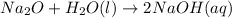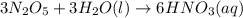Chemistry, 08.07.2021 16:00 myhomeacc32
Predict the products of each reaction, and whether the solution at equilibrium will be acidic, basic, or neutral.1. P4O10 + 6H2O (l)>2. Na2O + H2O(l) >3. N2O5 + 3H2O (l)>

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

Chemistry, 22.06.2019 23:00
What is the most common reason for matter changing its state?
Answers: 1

Chemistry, 22.06.2019 23:30
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3
You know the right answer?
Predict the products of each reaction, and whether the solution at equilibrium will be acidic, basic...
Questions









Chemistry, 20.09.2020 17:01







Mathematics, 20.09.2020 17:01




History, 20.09.2020 17:01