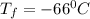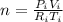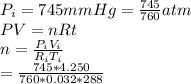A gas sample has a volume of 4250 mL at 15 degree Celsius and 745 mmHg. What is the final temperature, in degree Celsius, after the sample is transferred to a different container with a volume of 2.50 L and a pressure of 1.20 atm if the amount of gas does not change?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:10
Which of these conditions most likely produces an unstable isotope?
Answers: 2

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2
You know the right answer?
A gas sample has a volume of 4250 mL at 15 degree Celsius and 745 mmHg. What is the final temperatur...
Questions






Mathematics, 28.10.2020 17:10




Mathematics, 28.10.2020 17:10





Chemistry, 28.10.2020 17:10

Mathematics, 28.10.2020 17:10


English, 28.10.2020 17:10