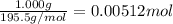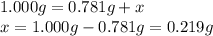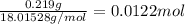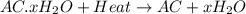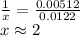A sample of unknown hydrate, AC-XH20, has a mass of 1.000 g before heating and a
mass of 0.781 g after heating. If the molar mass of the anhydrous compound (AC) is
195.5 g/mol, what is the water of crystallization for the formula of the unknown
hydrate?
Type your work for partial credit.
Answer choices: 2, 3, 5, or 6.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2

Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2
You know the right answer?
A sample of unknown hydrate, AC-XH20, has a mass of 1.000 g before heating and a
mass of 0.781 g af...
Questions

Mathematics, 20.09.2020 07:01



Mathematics, 20.09.2020 07:01

Business, 20.09.2020 07:01

English, 20.09.2020 07:01

Mathematics, 20.09.2020 07:01


Mathematics, 20.09.2020 07:01

Mathematics, 20.09.2020 07:01

World Languages, 20.09.2020 07:01


Biology, 20.09.2020 07:01


English, 20.09.2020 07:01



Mathematics, 20.09.2020 07:01

Mathematics, 20.09.2020 07:01