Chemistry, 08.07.2021 21:50 jjiopppotdd5638
For a reaction, AH = 176 kJ/mol and A SO = 0.285 kJ/(K•mol). At what
temperatures is this reaction spontaneous?
A. At no temperature
B. T< 50 K
C. T>617 K
D. T< 617 K

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 23.06.2019 01:00
Heat energy, carbon dioxide, and water are released through which process? a. photosynthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1

You know the right answer?
For a reaction, AH = 176 kJ/mol and A SO = 0.285 kJ/(K•mol). At what
temperatures is this reaction...
Questions

Chemistry, 04.03.2020 23:29




Computers and Technology, 04.03.2020 23:29




Physics, 04.03.2020 23:29

Computers and Technology, 04.03.2020 23:29



Computers and Technology, 04.03.2020 23:29

English, 04.03.2020 23:29






Mathematics, 04.03.2020 23:29

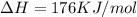
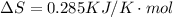
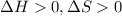
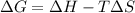
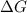 is negative, then the reaction is spontaneous.
is negative, then the reaction is spontaneous.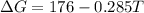
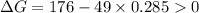
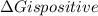 .Hence, the reaction is not spontaneous.
.Hence, the reaction is not spontaneous.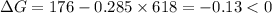
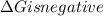 .Hence, the reaction is spontaneous.
.Hence, the reaction is spontaneous.