
Chemistry, 09.07.2021 03:20 yakshp3702
During a reaction, ΔH for reactants is −750 kJ/mol and ΔH for products is 920 kJ/mol. Which statement is correct about the reaction? (5 points)
Group of answer choices
It is endothermic because the energy required to break bonds in the reactants is less than the energy released when the products are formed.
It is endothermic because the energy required to break bonds in the reactants is greater than the energy released when the products are formed.
It is exothermic because the energy required to break bonds in the reactants is less than the energy released when the products are formed.
It is exothermic because the energy required to break bonds in the reactants is greater than the energy released when the products are formed.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 00:30
Which best describes why nh4+ can form an ionic bond with ci-?
Answers: 1

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3
You know the right answer?
During a reaction, ΔH for reactants is −750 kJ/mol and ΔH for products is 920 kJ/mol. Which statemen...
Questions


Social Studies, 09.10.2019 01:40

Social Studies, 09.10.2019 01:40

Mathematics, 09.10.2019 01:40

History, 09.10.2019 01:40

Health, 09.10.2019 01:40

History, 09.10.2019 01:50




Mathematics, 09.10.2019 01:50



History, 09.10.2019 01:50


Mathematics, 09.10.2019 01:50

Mathematics, 09.10.2019 01:50

History, 09.10.2019 01:50


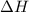 is always negative.
is always negative.

