
An element M reacts with another element X to form 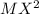 . In terms of loss or gain of electron, identify the element oxidized and reduced.
I need help in understanding this question, I know what a redox reaction is in terms of loss or gain of electron. However, I am not able to solve this question. I think that's maybe because the oxidation state or valency is not given for the unknown elements. Like, how do I know if the element is gaining or loosing electron when I don't know it's valency.
. In terms of loss or gain of electron, identify the element oxidized and reduced.
I need help in understanding this question, I know what a redox reaction is in terms of loss or gain of electron. However, I am not able to solve this question. I think that's maybe because the oxidation state or valency is not given for the unknown elements. Like, how do I know if the element is gaining or loosing electron when I don't know it's valency.
I repeat, I need to UNDERSTAND this question, I already know the answer but am just confused about how to solve it...

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Different isotopes indicate that an element will have different numbers of
Answers: 2

Chemistry, 21.06.2019 22:10
Here’s one way to follow the scientific method. place the missing steps in the correct position in the process
Answers: 1

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1
You know the right answer?
An element M reacts with another element X to form . In terms of loss or gain of electron, identify...
Questions

English, 04.12.2020 21:50


Mathematics, 04.12.2020 21:50

Mathematics, 04.12.2020 21:50







Physics, 04.12.2020 21:50



Mathematics, 04.12.2020 21:50

Mathematics, 04.12.2020 21:50

Social Studies, 04.12.2020 21:50


Mathematics, 04.12.2020 21:50

Mathematics, 04.12.2020 21:50

Mathematics, 04.12.2020 21:50



