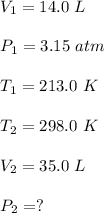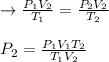Chemistry, 09.07.2021 20:20 yazmincruz3766
A gas is heated from 213.0 K to 298.0 K and the volume is increased from 14.0 liters to 35.0 liters by moving a large piston within a cylinder. If the original pressure was 3.15 atm, what would the final pressure be?
Include the following with your
Which Gas Law did you use?
The numerical answer to the question.
An explanation of the correct number of significant figures you will use for the numerical answer.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 21:00
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 22.06.2019 23:00
In the reaction h2co3 (aq) + 3nh3 (aq) = 2 nh4+ (aq) + co3 2-, how many electrons are transferred?
Answers: 3
You know the right answer?
A gas is heated from 213.0 K to 298.0 K and the volume is increased from 14.0 liters to 35.0 liters...
Questions



Biology, 07.10.2020 07:01


Mathematics, 07.10.2020 07:01


English, 07.10.2020 07:01

Biology, 07.10.2020 07:01

Mathematics, 07.10.2020 07:01


History, 07.10.2020 07:01


Business, 07.10.2020 07:01


Mathematics, 07.10.2020 07:01



Mathematics, 07.10.2020 07:01


Computers and Technology, 07.10.2020 07:01