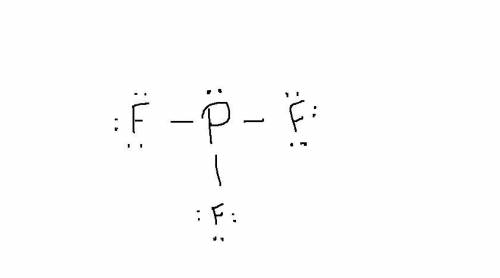Chemistry, 10.07.2021 01:20 jellyangie1
Write a Lewis structure for the phosphorus trifluoride molecule, PF3. Draw the Lewis dot structure for PF3. Draw the molecule by placing atoms on the grid and connecting them with bonds. Include all lone pairs of electrons.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:40
Which of the following pressures is equal to 760 mm hg? 2.0 atm 101.3 pa 101,300 kpa 101,300 pa
Answers: 2

Chemistry, 21.06.2019 23:00
Write a brief passage describing a neutral atom of nitrogen-14 (n-14). describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. use the periodic table to you. 14 protons and eletrons since its a neutral atom
Answers: 1

Chemistry, 23.06.2019 05:30
What is the morality of 2.50 l of solution that contains 1.85 mol of anhydrous sodium tetraborate?
Answers: 1

Chemistry, 23.06.2019 06:30
Acertain atom has 22 protons and 19 electrons. this atom loses an electron. the net charge on the atom is now 4+1+01-4-. if this same atom with 22 protons and 19 electrons were to gain 3 electrons, the net charge on the atom would be 3+2+02-3-.
Answers: 1
You know the right answer?
Write a Lewis structure for the phosphorus trifluoride molecule, PF3. Draw the Lewis dot structure f...
Questions

World Languages, 26.10.2020 20:50

Mathematics, 26.10.2020 20:50



Mathematics, 26.10.2020 20:50

History, 26.10.2020 20:50

Health, 26.10.2020 20:50

History, 26.10.2020 20:50

Mathematics, 26.10.2020 20:50



Geography, 26.10.2020 20:50

Biology, 26.10.2020 20:50

English, 26.10.2020 20:50

Mathematics, 26.10.2020 20:50

History, 26.10.2020 20:50


English, 26.10.2020 20:50


Health, 26.10.2020 20:50