Chemistry, 10.07.2021 04:10 preguntassimples
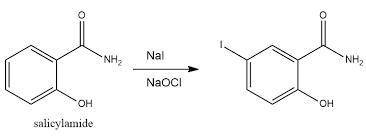
To conduct the synthesis of iodosalicylamide, Edward used 1.07 g of salicylamide (MW: 137.14 g/mol) and 1.68 g of sodium iodide (MW:149.89 g/mol). Assuming the reaction yield is 100%, how many grams of iodosalicylamide (MW:263.03 g/mol) would be formed

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1


Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3
You know the right answer?
To conduct the synthesis of iodosalicylamide, Edward used 1.07 g of salicylamide (MW: 137.14 g/mol)...
Questions

Mathematics, 08.04.2021 18:50

Physics, 08.04.2021 18:50



Mathematics, 08.04.2021 18:50

Physics, 08.04.2021 18:50


Mathematics, 08.04.2021 18:50

Health, 08.04.2021 18:50

Mathematics, 08.04.2021 18:50


Geography, 08.04.2021 18:50

Mathematics, 08.04.2021 18:50




Mathematics, 08.04.2021 18:50


Mathematics, 08.04.2021 18:50

Mathematics, 08.04.2021 18:50