
Chemistry, 11.07.2021 07:40 tommyaberman
How many moles of KClO3 must decompose in order to produce 9 moles of oxygen gas (O2) using the following reaction by completing the t-chart below.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 09:20
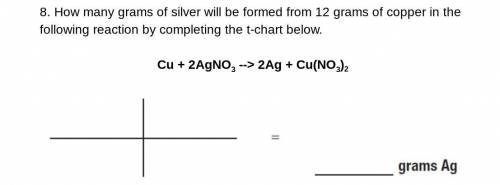
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2
You know the right answer?
How many moles of KClO3 must decompose in order to produce 9 moles of oxygen gas (O2) using the foll...
Questions

English, 14.12.2019 07:31



Social Studies, 14.12.2019 07:31


Mathematics, 14.12.2019 07:31

Social Studies, 14.12.2019 07:31


Physics, 14.12.2019 07:31

Mathematics, 14.12.2019 07:31

Mathematics, 14.12.2019 07:31




Mathematics, 14.12.2019 07:31

Mathematics, 14.12.2019 07:31


Mathematics, 14.12.2019 07:31

History, 14.12.2019 07:31

Physics, 14.12.2019 07:31

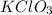 will be decomposed.
will be decomposed.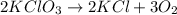
 of
of 

