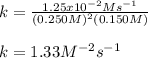NO2 (g) reacts with O2 4 NO2 (g) + O2(g) to produce N2 O5 (g) as shown by the equation below: (g) = 2 N2 O5 (g).
Experimentally the rate orders were determined and rate law written as shown below: Rate = k [NO2]2[O2 ].
Calculate the value of k if the initial concentration of NO2 was 0.250 M and initial
concentration of O2 (g) was 0.150 M. The initial rate was 1.25 x 10-2 M. s-1 .

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Which term describes a fracture in the earth at which land stays in the same place? a. joint b. fault c. split d. hinge
Answers: 1

Chemistry, 22.06.2019 01:30
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 21:30
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3

You know the right answer?
NO2 (g) reacts with O2 4 NO2 (g) + O2(g) to produce N2 O5 (g) as shown by the equation below: (g) =...
Questions

Arts, 19.09.2019 01:00



History, 19.09.2019 01:00



History, 19.09.2019 01:00

Mathematics, 19.09.2019 01:00


Biology, 19.09.2019 01:00

Mathematics, 19.09.2019 01:00






Mathematics, 19.09.2019 01:00



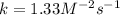
![k=\frac{r}{[NO_2]^2[O_2]}](/tpl/images/1392/3789/849e7.png)