
Chemistry, 13.07.2021 04:00 erikloza12pdidtx
Solutions of Cu2+ turn blue litmus red because of the equilibrium: Cu(H2O)62+(aq) + H2O(l) ↔ Cu(H2O)5(OH)+(aq) + H3O+(aq) for which Ka = 1.0 x 10-8. Calculate the pH of 0.10 M Cu(NO3)2(aq).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2


Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1
You know the right answer?
Solutions of Cu2+ turn blue litmus red because of the equilibrium: Cu(H2O)62+(aq) + H2O(l) ↔ Cu(H2O)...
Questions


English, 12.10.2020 20:01

English, 12.10.2020 20:01


Mathematics, 12.10.2020 20:01

Computers and Technology, 12.10.2020 20:01





Social Studies, 12.10.2020 20:01



Mathematics, 12.10.2020 20:01


Business, 12.10.2020 20:01

Mathematics, 12.10.2020 20:01

Mathematics, 12.10.2020 20:01

Mathematics, 12.10.2020 20:01

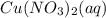 is 4.49.
is 4.49.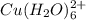 = 0.10 M
= 0.10 M
![Cu(H_{2}O)^{2+}_{6} \rightleftharpoons [Cu(H_{2}O)_{5}(OH)]^{+} + H_{3}O^{+}](/tpl/images/1393/0831/fa913.png)
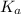 is very small. So, it is assumed that the compound will dissociate very less. Hence, x << 0.10 M.
is very small. So, it is assumed that the compound will dissociate very less. Hence, x << 0.10 M.![K_{a} = \frac{[Cu(H_{2}O)^{2+}_{6}][H_{3}O^{+}]}{[Cu(H_{2}O)^{2+}_{6}]}\\1.0 \times 10^{-8} = \frac{x \times x}{0.10}\\x = 3.2 \times 10^{-5}](/tpl/images/1393/0831/ba8e8.png)
![[H_{3}O^{+}] = 3.2 \times 10^{-5}](/tpl/images/1393/0831/f5dd0.png)
![pH = -log [H^{+}]](/tpl/images/1393/0831/8d00e.png)
![pH = -log [H^{+}]\\= - log (3.2 \times 10^{-5})\\= 4.49](/tpl/images/1393/0831/392b2.png)



