
Octane (C8H18) is a component of gasoline that burns according to the following equation: C8H18(l) + 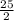 O2(g) → 8CO2(g) + 9H2O(g) ΔH∘rxn = −5074.1 kJ
What mass of octane (in g) is required to produce 8210 kJ of heat?
O2(g) → 8CO2(g) + 9H2O(g) ΔH∘rxn = −5074.1 kJ
What mass of octane (in g) is required to produce 8210 kJ of heat?
Express your answer to three significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2

Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2

Chemistry, 23.06.2019 09:00
Describe the process that was used in this lab to create magnesium oxide, specifically identifying the type of chemical reaction. explain why the product had a higher mass than the reactant, and how this relates to conservation of matter.
Answers: 2
You know the right answer?
Octane (C8H18) is a component of gasoline that burns according to the following equation: C8H18(l) +...
Questions


Biology, 31.08.2021 21:30

Mathematics, 31.08.2021 21:30



Mathematics, 31.08.2021 21:30



Mathematics, 31.08.2021 21:30



Mathematics, 31.08.2021 21:30

Mathematics, 31.08.2021 21:30

History, 31.08.2021 21:30

Biology, 31.08.2021 21:30

Mathematics, 31.08.2021 21:30

Physics, 31.08.2021 21:30


History, 31.08.2021 21:30



