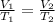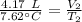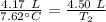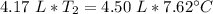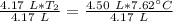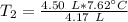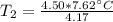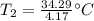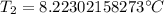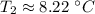Chemistry, 14.07.2021 04:20 Sparkledog
A 4.17 L volume of oxygen gas measured at 7.62 °C is expanded to a new volume of 4.50 L. Calculate the temperature (in oC) of the gas at the higher volume, assuming no change in pressure.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Which compounds have the empirical formula ch2o? a.c2h4o2 b.c3h6o3 c.ch2o2 d.c5h10o5 e.c6h12o6
Answers: 3

Chemistry, 22.06.2019 04:00
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1
You know the right answer?
A 4.17 L volume of oxygen gas measured at 7.62 °C is expanded to a new volume of 4.50 L. Calculate t...
Questions

Social Studies, 18.11.2020 21:50

Mathematics, 18.11.2020 21:50

History, 18.11.2020 21:50



Chemistry, 18.11.2020 21:50


Mathematics, 18.11.2020 21:50

Mathematics, 18.11.2020 21:50

Mathematics, 18.11.2020 21:50



Mathematics, 18.11.2020 21:50



Mathematics, 18.11.2020 21:50

Mathematics, 18.11.2020 21:50


Chemistry, 18.11.2020 21:50

Geography, 18.11.2020 21:50