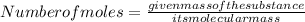Chemistry, 14.07.2021 04:50 cadenbukvich9923
Which of the following steps correctly converts 1.25 moles of fluorine to an equivalent mass of fluorine in grams? (5 points)
Add 1.25 to the atomic mass of fluorine.
Divide the atomic mass of fluorine by 1.25.
Subtract 1.25 from the atomic mass of fluorine.
Multiply the atomic mass of fluorine by 1.25.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2
You know the right answer?
Which of the following steps correctly converts 1.25 moles of fluorine to an equivalent mass of fluo...
Questions



Chemistry, 27.07.2019 11:30



Mathematics, 27.07.2019 11:30

English, 27.07.2019 11:30


Chemistry, 27.07.2019 11:30

History, 27.07.2019 11:30

Spanish, 27.07.2019 11:30

English, 27.07.2019 11:30

Mathematics, 27.07.2019 11:30

Mathematics, 27.07.2019 11:30




History, 27.07.2019 11:30


Health, 27.07.2019 11:30