
Chemistry, 14.07.2021 19:00 ChloeN8912
. The Ksp of barium sulfate is 1.1 × 10–10. What is the sulfate-ion concentration of a 1.0-L saturated solution of BaSO4 to which 0.025 mol of Ba(NO3)2 is added? 4.4 × 10–9M 1.0 × 10–5M 6.6 × 10–5M 2.8 × 10–12M

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2

Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3

You know the right answer?
. The Ksp of barium sulfate is 1.1 × 10–10. What is the sulfate-ion concentration of a 1.0-L saturat...
Questions





Law, 13.04.2021 02:10



Mathematics, 13.04.2021 02:10


English, 13.04.2021 02:10

Mathematics, 13.04.2021 02:10







Biology, 13.04.2021 02:10


![[SO_4^{2-}]=4.4*10^{-9}M](/tpl/images/1394/0680/f5cea.png)
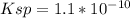
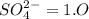
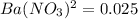
![Ksp=[Ba^{2+}][SO_4^{2-}]](/tpl/images/1394/0680/8dfdf.png)
![1.1*10^{-10}=[0.025][SO_4^{2-}]](/tpl/images/1394/0680/613b0.png)


