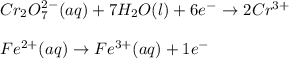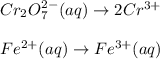Chemistry, 15.07.2021 17:30 gfdsgfd5654
Write balanced half-reactions for the following redox reaction: Cr2O2-7(aq) 7H2O(l) 6Fe2 (aq) 2Cr3 (aq) 14OH-(aq) 6Fe3 (aq)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 22:30
Rank the four gases (air, exhaled air, gas produced from from decomposition of h2o2, gas from decomposition of nahco3) in order of increasing concentration of co2
Answers: 1

Chemistry, 23.06.2019 01:00
Which of the following is the molecular formula for a simple sugar? a. cooh b. h2o c. oh d. c6h12o6
Answers: 1
You know the right answer?
Write balanced half-reactions for the following redox reaction: Cr2O2-7(aq) 7H2O(l) 6Fe2 (aq) 2Cr3 (...
Questions


Mathematics, 07.07.2019 07:30

Advanced Placement (AP), 07.07.2019 07:30

Chemistry, 07.07.2019 07:30

Advanced Placement (AP), 07.07.2019 07:30

Advanced Placement (AP), 07.07.2019 07:30

Advanced Placement (AP), 07.07.2019 07:30

Advanced Placement (AP), 07.07.2019 07:30


Mathematics, 07.07.2019 07:30




Mathematics, 07.07.2019 07:30




History, 07.07.2019 07:30