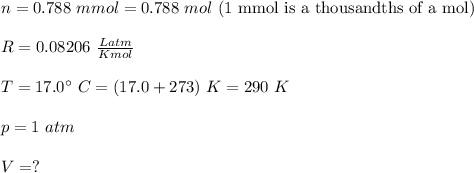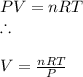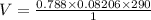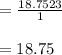Chemistry, 15.07.2021 21:00 bellapimienta8
A reaction at evolves of dinitrogen monoxide gas. Calculate the volume of dinitrogen monoxide gas that is collected. You can assume the pressure in the room is exactly . Be sure your answer has the correct number of significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Often on a topographic map, every fifth contour line is darkened. what is this line called? a. key b.slope c.benchmark d. index contour
Answers: 1

Chemistry, 22.06.2019 18:00
Answer asap need it by wednesday morning carry out the following calculations on ph and ka of from data. i. calculate the ph of 0.02m hcl ii. calculate the ph of 0.036m naoh iii. calculate the ph of 0.36m ca(oh)2 iv. calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 v. calculate ka for weak acid ha which has a ph of 3.65 at 0.30m concentration vi. calculate the ka of a solution made by mixing 15.0 cm3 0.2m ha and 60.0 cm3 0.31m a-. [ph= 3.80] vii. calculate the ph of a solution made by mixing 15.0 cm3 0.1m naoh and 35.0 cm3 0.2m hcooh. [ka = 1.82 x 10-4 m]
Answers: 1


You know the right answer?
A reaction at evolves of dinitrogen monoxide gas. Calculate the volume of dinitrogen monoxide gas th...
Questions

Mathematics, 30.01.2020 13:02

Mathematics, 30.01.2020 13:02

Mathematics, 30.01.2020 13:02



Advanced Placement (AP), 30.01.2020 13:02



Computers and Technology, 30.01.2020 13:02

Mathematics, 30.01.2020 13:02


Mathematics, 30.01.2020 13:02


Geography, 30.01.2020 13:02

English, 30.01.2020 13:03

Advanced Placement (AP), 30.01.2020 13:03

Biology, 30.01.2020 13:03

Mathematics, 30.01.2020 13:03

Mathematics, 30.01.2020 13:03

 ".
".