
Chemistry, 16.07.2021 03:40 abbypoletick
The reversible reaction: 2SO2(g) O2(g) darrow-tn. gif 2SO3(g) has come to equilibrium in a vessel of specific volume at a given temperature. Before the reaction began, the concentrations of the reactants were 0.060 mol/L of SO2 and 0.050 mol/L of O2. After equilibrium is reached, the concentration of SO3 is 0.040 mol/L. What is the equilibrium concentration of O2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

You know the right answer?
The reversible reaction: 2SO2(g) O2(g) darrow-tn. gif 2SO3(g) has come to equilibrium in a vessel of...
Questions






History, 18.11.2019 19:31














![[O_2]_{eq}=0.030M](/tpl/images/1395/0026/cfd77.png)
![[O_2]_{eq}=0.050M-x](/tpl/images/1395/0026/a8660.png)
 can be found considering the equilibrium of SO3:
can be found considering the equilibrium of SO3:![[SO_3]_{eq}=2x=0.040M](/tpl/images/1395/0026/c4625.png)
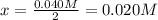
![[O_2]_{eq}=0.050M-0.020M=0.030M](/tpl/images/1395/0026/b7171.png)


