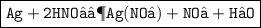Write balanced chemical equations for the following reactions.
Carbon disulfide + oxygen gas gives carbon dioxide + sulfur dioxide.
Silver + nitric acid gives silver nitrate + nitrogen dioxide + water.
If you can explain how to do each it will be greatly appreciated.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Select all that apply. a beta particle: is electromagnetic energy is an electron has zero charge is emitted from the nucleus has a +2 charge has a -1 charge
Answers: 1

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 16:00
Which process transfers heat from inside earth to its surface? convection currents in mantle pulling away of tectonic plates drawing in of tectonic plates convection currents in crust
Answers: 1
You know the right answer?
Write balanced chemical equations for the following reactions.
Carbon disulfide + oxygen gas gives...
Questions


Health, 15.10.2020 09:01



Mathematics, 15.10.2020 09:01

Mathematics, 15.10.2020 09:01

Mathematics, 15.10.2020 09:01



Chemistry, 15.10.2020 09:01

English, 15.10.2020 09:01


Mathematics, 15.10.2020 09:01

Mathematics, 15.10.2020 09:01

English, 15.10.2020 09:01

Mathematics, 15.10.2020 09:01

Mathematics, 15.10.2020 09:01


Mathematics, 15.10.2020 09:01