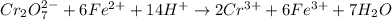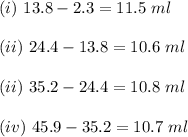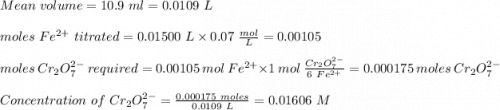Chemistry, 16.07.2021 20:00 jcazaresroman7308
ProblemWhat is the concentration of a tin(ll) chloride solution prepared from a sample of tin ore?Experimental DesignThe potassium dichromate solution is first standardized by titration with 15.00 mL of an acidified 0.07 mol/L solution of the primary standard, iron(II) ammonium sulfate-6-water. The standardized dichromate solution is then titrated against a sample of the acidified tin(II) chloride solution (You will do this step in the next question). Evidence TITRATION OF IRON(lI) SOLUTION(volume of K2Cr2O7(aq) required to react with 15.00 ml of 0.07 mol/L Fe2+(aq)) Trial1234Final buretreading(ml) 13.824.435.245.9Initial buretreading (ml) 2.313.824.435.2Find the concentration of the Cr2O72-(aq) in mol/L: (give your answer to 4 decimal places)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which feature do highland climates have that lower elevation areas do not?
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3


Chemistry, 22.06.2019 22:00
11) burning your hand when accidentally touching a hot plate is an example of which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 2
You know the right answer?
ProblemWhat is the concentration of a tin(ll) chloride solution prepared from a sample of tin ore?Ex...
Questions


Geography, 03.08.2019 18:00



History, 03.08.2019 18:00

History, 03.08.2019 18:00




History, 03.08.2019 18:00


Mathematics, 03.08.2019 18:00

Mathematics, 03.08.2019 18:00



English, 03.08.2019 18:00


Biology, 03.08.2019 18:00