
Chemistry, 17.07.2021 01:00 heyitstierney5610
Use the problem below to answer the question:
34 grams of carbon reacted with an unlimited amount of H2O. The reaction is:
C + H2O → CO + H2
The atomic mass of C is 12.01 g/mole. The atomic mass of H2 is 2.016 g/mole. Finish the problem by choosing the correct format for dimensional analysis.
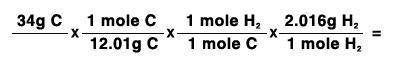
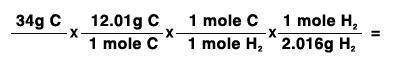
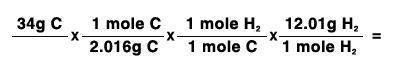

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:50
How many grams of oxygen gas are contained in a 15 l sample at 1.02 atm and 28°c? show your work.
Answers: 1

Chemistry, 22.06.2019 12:40
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2

Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3

Chemistry, 22.06.2019 19:00
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1
You know the right answer?
Use the problem below to answer the question:
34 grams of carbon reacted with an unlimited amount o...
Questions

History, 05.06.2020 04:01

Physics, 05.06.2020 04:01


Mathematics, 05.06.2020 04:01



English, 05.06.2020 04:01


Mathematics, 05.06.2020 04:01

Social Studies, 05.06.2020 04:01










Mathematics, 05.06.2020 04:01



