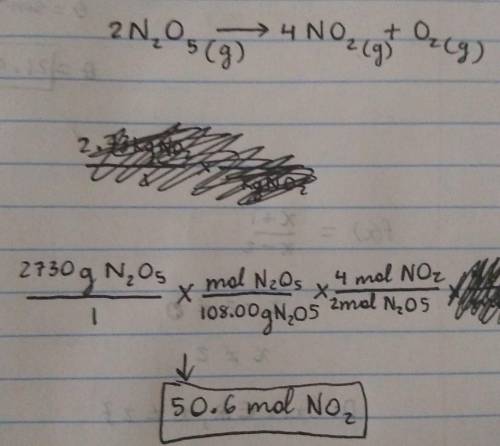Chemistry, 19.07.2021 03:40 anonymous1813
Calculate how many moles of NO2
form when each quantity of reactant completely reacts via the following reaction:
2N2O5(g)→4NO2(g)+O2(g)
2.73 kg N2O5
Express your answer using three significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 23.06.2019 03:00
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 1
You know the right answer?
Calculate how many moles of NO2
form when each quantity of reactant completely reacts via the follo...
Questions




Mathematics, 30.01.2021 01:00


Advanced Placement (AP), 30.01.2021 01:00


Mathematics, 30.01.2021 01:00

Mathematics, 30.01.2021 01:00

Mathematics, 30.01.2021 01:00

History, 30.01.2021 01:00

Geography, 30.01.2021 01:00

Mathematics, 30.01.2021 01:00


Social Studies, 30.01.2021 01:00

Arts, 30.01.2021 01:00

Chemistry, 30.01.2021 01:00

Mathematics, 30.01.2021 01:00

Health, 30.01.2021 01:00