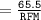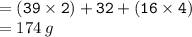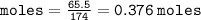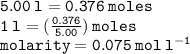Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1


Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

You know the right answer?
Calculate the molarity of a solution consisting of 65.5 g of K2S0 4 in 5.00 L of solution. ...
Questions


Computers and Technology, 12.08.2020 08:01












Mathematics, 12.08.2020 08:01