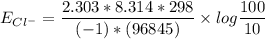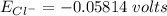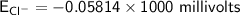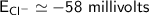The Nernst equation at 20oC is:
Eion= 58 millvolts/z. [log10 (ion)out/(ion)in]
Calculat...

Chemistry, 19.07.2021 17:10 shortty1111
The Nernst equation at 20oC is:
Eion= 58 millvolts/z. [log10 (ion)out/(ion)in]
Calculate the equilibrium potential for Cl- if the concentration of Cl- outside of the cell is 100 and the concentration inside of the cell is 10 mmol/liter.
a. 58 millivolts
b. +58 millivolts
c. -116 millivolts
d. 0

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3

Chemistry, 23.06.2019 03:50
What is the equation fort the alkaline zinc/manganese dioxide cell. a) anode b)cathode c)overall equations.
Answers: 2
You know the right answer?
Questions

Mathematics, 24.08.2021 14:30


Arts, 24.08.2021 14:40

Mathematics, 24.08.2021 14:40

Social Studies, 24.08.2021 14:40

English, 24.08.2021 14:40

Arts, 24.08.2021 14:40

English, 24.08.2021 14:40

History, 24.08.2021 14:40



Business, 24.08.2021 14:40




Business, 24.08.2021 14:40

Business, 24.08.2021 14:40

Biology, 24.08.2021 14:40

Mathematics, 24.08.2021 14:40

History, 24.08.2021 14:40

![E_{ion} = 58 millivolts /z \Big[ log_{10} \Big( \dfrac{[ion]_{out}}{[ion]_{in}}\Big) \Big]}](/tpl/images/1396/2001/4bd00.png)
![E_{Cl^-} = \dfrac{2.303*R*T}{ZF} \times log \dfrac{[Cl^-]_{out}} {[Cl^-]_{in}}](/tpl/images/1396/2001/9486b.png)