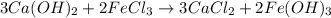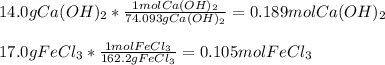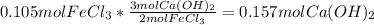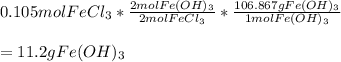Chemistry, 20.07.2021 04:10 kashmoney8690
A chemist dissolves 14.0 g of calcium hydroxide in one beaker of water, and 17.0 g of iron(III) chloride
in a second beaker of water. Everything dissolves.
When the two solutions are poured together, solid iron(III) hydroxide precipitates.
1. Write a balanced molecular equation.
2. Determine the identity of the limiting reactant.
3. Predict the mass of iron(III) hydroxide product.

Answers: 1


Another question on Chemistry

Chemistry, 20.06.2019 18:02
When undergoing chemical reactions where does the reactant combine to the enzyme?
Answers: 1

Chemistry, 21.06.2019 21:00
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1

Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1
You know the right answer?
A chemist dissolves 14.0 g of calcium hydroxide in one beaker of water, and 17.0 g of iron(III) chlo...
Questions



Chemistry, 07.01.2021 01:10


Physics, 07.01.2021 01:10


Mathematics, 07.01.2021 01:10



German, 07.01.2021 01:10


Mathematics, 07.01.2021 01:10



English, 07.01.2021 01:10

Mathematics, 07.01.2021 01:10

Mathematics, 07.01.2021 01:10

English, 07.01.2021 01:10

Mathematics, 07.01.2021 01:10

English, 07.01.2021 01:10