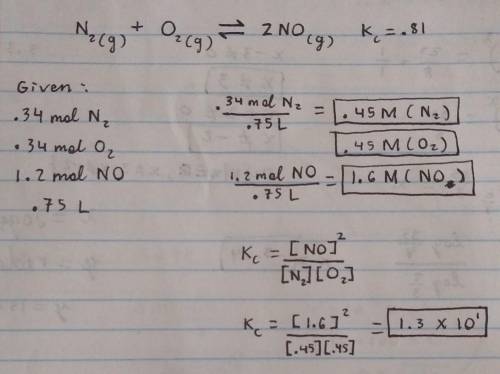Chemistry, 21.07.2021 01:20 Goodgirlkl12k
The decomposition of nitric oxide to molecular nitrogen and oxygen occurs at high temperatures according to the reaction:
N2(g)+O2(g)⟺2NO(g)Kc=0.81
At the start of the reaction, 0.34 mol N2, 0.34 mol O2 and 1.2 mol NO are introduced into a 0.75 L reaction chamber and allowed to equilibrate.
Required:
What is the concentration of NO (in M) at equilibrium?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1


Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

Chemistry, 22.06.2019 21:00
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1
You know the right answer?
The decomposition of nitric oxide to molecular nitrogen and oxygen occurs at high temperatures accor...
Questions

Computers and Technology, 10.03.2020 06:04






Mathematics, 10.03.2020 06:04

Mathematics, 10.03.2020 06:04








Social Studies, 10.03.2020 06:04

Mathematics, 10.03.2020 06:04