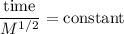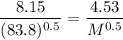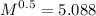Chemistry, 21.07.2021 19:50 alivas6618
A sample of Kr gas is observed to effuse through a pourous barrier in 8.15 minutes. Under the same conditions, the same number of moles of an unknown gas requires 4.53 minutes to effuse through the same barrier. The molar mass of the unknown gas is g/mol.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 23.06.2019 05:00
How many atomic mass units are equal to 1.672×10−24 g of protons?
Answers: 3

Chemistry, 23.06.2019 08:00
What is the temperature in kelvin of a gas if it is allowed to expand from 1.50 l to 4.50 l? the initial temperature is 10.0°c and pressure is constant throughout the change. which equation should you use? t2= v2/v1 t1 what is the final temperature? ⇒ 849 k these are the answers.
Answers: 1

Chemistry, 24.06.2019 04:20
The maximum amounts of lead and copper allowed in drinking water are 0.015 mg/kg for lead and 1.3 mg/kg for copper. tell the maximum amount of copper (in grams) allowed in 100 g of water.
Answers: 3
You know the right answer?
A sample of Kr gas is observed to effuse through a pourous barrier in 8.15 minutes. Under the same c...
Questions

English, 25.02.2021 21:40

History, 25.02.2021 21:40


Geography, 25.02.2021 21:40


Mathematics, 25.02.2021 21:40

Chemistry, 25.02.2021 21:40

Mathematics, 25.02.2021 21:40

History, 25.02.2021 21:40



Health, 25.02.2021 21:40

Mathematics, 25.02.2021 21:40


Mathematics, 25.02.2021 21:40

Chemistry, 25.02.2021 21:40


Mathematics, 25.02.2021 21:40

Mathematics, 25.02.2021 21:40

Chemistry, 25.02.2021 21:50