
Chemistry, 21.07.2021 21:20 denisebaslee15
What direction would equilibrium moves towards based on the following if we increased the volume of the container.
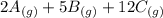 ↔
↔ 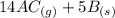
Answer choices:
a) reactants
b) no change
c) products
d) decrease in volume
Please help!

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1
You know the right answer?
What direction would equilibrium moves towards based on the following if we increased the volume of...
Questions





Biology, 28.03.2020 08:22

Mathematics, 28.03.2020 08:22



Mathematics, 28.03.2020 08:22

Mathematics, 28.03.2020 08:22


Mathematics, 28.03.2020 08:24




English, 28.03.2020 08:37



Chemistry, 28.03.2020 08:37




