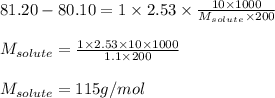Chemistry, 21.07.2021 21:30 mosleykimberly944
10g of a non-volatile and non-dissociating solute is dissolved in 200g of benzene.
The resulting solution boils At temperature of 81.20oC. Find the molar mass of solute.
Given that the BP of pure benzene is 80.10oC and Its elevation boiling point constant = 2.53 oC/m.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3


Chemistry, 23.06.2019 06:30
How can the number of core electrons be determined from the periodic table
Answers: 1

Chemistry, 23.06.2019 07:00
0.88 moles of n2o5 (g) was placed in a sealed 1.00 l vessel. calculate the equilibrium concentration of n2o5. no2, and o2 and the equilibrium constant after equilibrium has been reached by 65.0% of the n2o5 decomposing.
Answers: 1
You know the right answer?
10g of a non-volatile and non-dissociating solute is dissolved in 200g of benzene.
The resulting so...
Questions



Mathematics, 22.01.2021 21:50

Mathematics, 22.01.2021 21:50

Social Studies, 22.01.2021 21:50

Mathematics, 22.01.2021 21:50





Biology, 22.01.2021 21:50



Mathematics, 22.01.2021 21:50


Mathematics, 22.01.2021 21:50

Mathematics, 22.01.2021 21:50


Mathematics, 22.01.2021 21:50

Biology, 22.01.2021 21:50

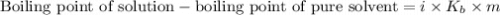
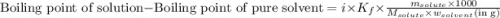 ......(1)
......(1)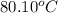
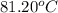
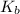 = Boiling point elevation constant =
= Boiling point elevation constant = 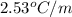
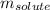 = Given mass of solute = 10 g
= Given mass of solute = 10 g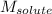 = Molar mass of solute = ? g/mol
= Molar mass of solute = ? g/mol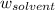 = Mass of solvent = 200 g
= Mass of solvent = 200 g