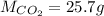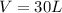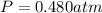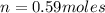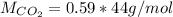Carbon dioxide gas is collected at 27.0 oC in an evacuated flask with a measured volume of 30.0L. When all the gas has been collected, the pressure in the flask is measured to be 0.480atm. Calculate the mass and number of moles of carbon dioxide gas that were collected.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3



Chemistry, 22.06.2019 23:00
What is the formula of the ionic compound composed of calcium cations and chloride anions
Answers: 1
You know the right answer?
Carbon dioxide gas is collected at 27.0 oC in an evacuated flask with a measured volume of 30.0L. Wh...
Questions



Biology, 15.05.2021 02:10

Social Studies, 15.05.2021 02:10

Mathematics, 15.05.2021 02:10

Biology, 15.05.2021 02:10




Mathematics, 15.05.2021 02:10

Mathematics, 15.05.2021 02:10


Mathematics, 15.05.2021 02:10

Mathematics, 15.05.2021 02:10

Biology, 15.05.2021 02:10

Geography, 15.05.2021 02:10

Mathematics, 15.05.2021 02:10


English, 15.05.2021 02:10

Mathematics, 15.05.2021 02:10