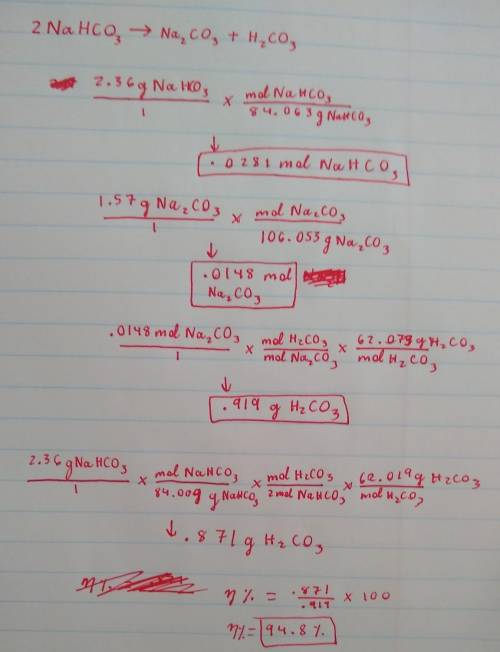ASAP
A 2.36-gram sample of NaHCO3 was completely decomposed in an experiment.
2NaHCO→ Na...

Chemistry, 24.07.2021 01:00 crookdamian21
ASAP
A 2.36-gram sample of NaHCO3 was completely decomposed in an experiment.
2NaHCO→ Na2CO3 + H2CO3
In this experiment, carbon dioxide and water vapors combine to form H2CO3. After decomposition, the Na2CO3 had a mass of 1.57 grams.
Determine the mass of the H2CO3 produced.
Calculate the percentage yield of H2CO3 for the reaction. Show your work or describe the calculation process in detail.
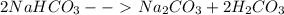

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 12:00
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3
You know the right answer?
Questions

History, 31.07.2019 18:00



History, 31.07.2019 18:00

Social Studies, 31.07.2019 18:00

Social Studies, 31.07.2019 18:00

History, 31.07.2019 18:00

Biology, 31.07.2019 18:00





Biology, 31.07.2019 18:00

Biology, 31.07.2019 18:00

Chemistry, 31.07.2019 18:00


Social Studies, 31.07.2019 18:00

Chemistry, 31.07.2019 18:00

Chemistry, 31.07.2019 18:00

History, 31.07.2019 18:00