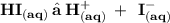Chemistry, 24.07.2021 17:00 biancasamadp3usfw
Complete the balanced dissociation equation for the compound below in aqueous solution. If the compound does not dissociate, write NR after the reaction arrow. HI (aq) -->

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1

Chemistry, 22.06.2019 19:10
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3

Chemistry, 23.06.2019 01:00
Which statement is true regarding the diagram of circle p? the sum of y and z must be 2x. the sum of y and z must be x. the difference of z and y must be 2x. the difference of z and y must be x
Answers: 1
You know the right answer?
Complete the balanced dissociation equation for the compound below in aqueous solution. If the compo...
Questions

Social Studies, 23.09.2020 16:01

Law, 23.09.2020 16:01


Mathematics, 23.09.2020 16:01

English, 23.09.2020 16:01



History, 23.09.2020 16:01

Advanced Placement (AP), 23.09.2020 16:01

Spanish, 23.09.2020 16:01

Chemistry, 23.09.2020 16:01


Mathematics, 23.09.2020 16:01