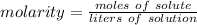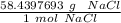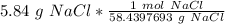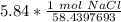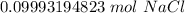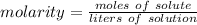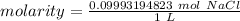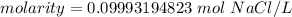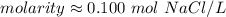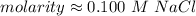Chemistry, 25.07.2021 04:00 brebun4742
A solution is made by dissolving 5.84 grams of NaCl in enough distilled water to give a final volume of 1.00 L. What is the molarity of the solution
Group of answer choices
0.0250 M
0.400 M
0.100 M
1.00 M

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3



Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
You know the right answer?
A solution is made by dissolving 5.84 grams of NaCl in enough distilled water to give a final volume...
Questions

Mathematics, 14.04.2021 18:30



Mathematics, 14.04.2021 18:30

Mathematics, 14.04.2021 18:30

Chemistry, 14.04.2021 18:30

Mathematics, 14.04.2021 18:30



Mathematics, 14.04.2021 18:30



Arts, 14.04.2021 18:30

Mathematics, 14.04.2021 18:30

Mathematics, 14.04.2021 18:30


Mathematics, 14.04.2021 18:30

Mathematics, 14.04.2021 18:30

Physics, 14.04.2021 18:30

Mathematics, 14.04.2021 18:30