
Chemistry, 25.07.2021 06:20 brooklynunderwood46
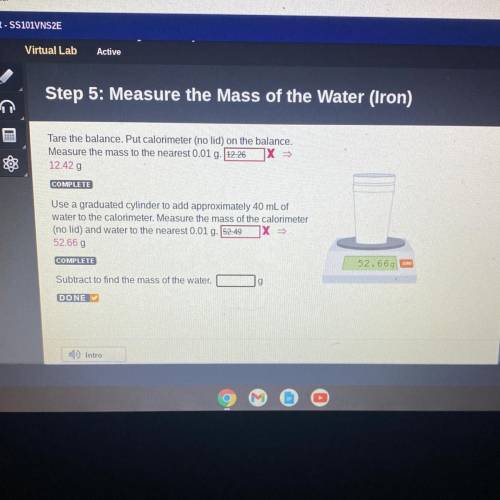
Step 5: Measure the Mass of the Water (Iron)
Tare the balance. Put calorimeter (no lid) on the balance.
Measure the mass to the nearest 0.01 g. 12.26 X
12.42 g
COMPLETE
Use a graduated cylinder to add approximately 40 mL of
water to the calorimeter. Measure the mass of the calorimeter
(no lid) and water to the nearest 0.01 g. 52.49
52.66 g
COMPLETE
52.669
Subtract to find the mass of the water.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

Chemistry, 23.06.2019 05:30
How many moles are in 1.26*10^24 particles in significant figures
Answers: 2

Chemistry, 23.06.2019 11:20
Match each state of matter with the statement that best describes it.
Answers: 1
You know the right answer?
Step 5: Measure the Mass of the Water (Iron)
Tare the balance. Put calorimeter (no lid) on the bala...
Questions

Biology, 05.05.2020 06:24

Physics, 05.05.2020 06:24

Arts, 05.05.2020 06:24

Biology, 05.05.2020 06:24

Geography, 05.05.2020 06:24







English, 05.05.2020 06:24





English, 05.05.2020 06:24






