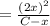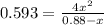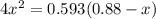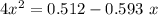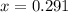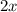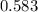Chemistry, 25.07.2021 20:20 marissalwilliams3
For the following reaction of N2O4, the equilibrium constant is 0.593 at a particular temperature.
N2O4(g) ⇌ 2 NO2(g)
If the initial concentration of N2O4 is 0.880M, what are the equilibrium concentrations?
Please show work!

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 23.06.2019 04:10
In an experiment, 45g of silicon tetrachloride are treated with 45ml of water. what is the theoretical yield in grams of hcl
Answers: 3

Chemistry, 23.06.2019 06:00
What volume of 0.500 mol/l hydrochloric acid, hci (aq) is required to react completely with 1.00 g of aluminum hydroxide, ai(oh)3 (s)?
Answers: 1

Chemistry, 23.06.2019 08:00
The goal of this experiment was to answer the question "what is the effect of a gas' temperature on its volume? " you formulated the hypothesis below. hypothesis: if a fixed amount of gas is heated, then the volume will increase because the heat will cause the molecules of gas to move faster and further apart. to test this hypothesis, you changed the of the gas between 0 and 100°c (273 and 373 k) and calculated the resulting of the gas.
Answers: 2
You know the right answer?
For the following reaction of N2O4, the equilibrium constant is 0.593 at a particular temperature....
Questions


Mathematics, 16.12.2020 23:40



History, 16.12.2020 23:40



History, 16.12.2020 23:40


Mathematics, 16.12.2020 23:40

Mathematics, 16.12.2020 23:40


Mathematics, 16.12.2020 23:40

Biology, 16.12.2020 23:40

Social Studies, 16.12.2020 23:40


Chemistry, 16.12.2020 23:40


Mathematics, 16.12.2020 23:40

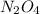 be "C".
be "C".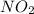 be "x".
be "x".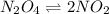
![K_c = \frac{[NO_2]^2}{[N_2O_4]}](/tpl/images/1399/4499/98df0.png)