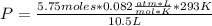A chemist is preparing to carry out a reaction that requires 5.75 moles of hydrogen gas. The chemist pumps the hydrogen into a 10.5 L rigid steel container at 20.0 °C. To what pressure, in kPa, must the hydrogen be compressed? (Show all work for full credit and circle your final answer) *

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1

Chemistry, 22.06.2019 10:10
When electrolyzing copper (ll) chloride, what reaction takes place at the anode? what reaction takes place at the cathode?
Answers: 1

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2

You know the right answer?
A chemist is preparing to carry out a reaction that requires 5.75 moles of hydrogen gas. The chemist...
Questions

Mathematics, 12.12.2020 16:50

Health, 12.12.2020 16:50

Advanced Placement (AP), 12.12.2020 16:50

Mathematics, 12.12.2020 16:50

Mathematics, 12.12.2020 16:50

History, 12.12.2020 16:50

Spanish, 12.12.2020 16:50




Biology, 12.12.2020 16:50

Mathematics, 12.12.2020 16:50

Mathematics, 12.12.2020 16:50

English, 12.12.2020 16:50

Arts, 12.12.2020 16:50


Computers and Technology, 12.12.2020 16:50

Social Studies, 12.12.2020 16:50


Mathematics, 12.12.2020 16:50

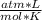 T= 20 C= 293 K (being 0 C= 273 K)
T= 20 C= 293 K (being 0 C= 273 K)