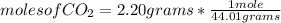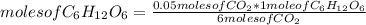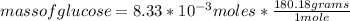Chemistry, 27.07.2021 14:00 joshvslogic341
The reaction for photosynthesis producing glucose sugar and oxygen gas is:
__CO2(g) + __H2O(l) UV/chlorophyl−→−−−−−−−−−−−−−− __C6H12O6(s) + __O2(g)
What is the mass of glucose (180.18 g/mol) produced from 2.20 g of CO2 (44.01 g/mol)?
a. 66.1 g C6H12O6
b. 396 g C6H12O6
c. 54.0 g C6H12O6
d. 1.50 g C6H12O6
e. 9.01 g C6H12O6

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2



You know the right answer?
The reaction for photosynthesis producing glucose sugar and oxygen gas is:
__CO2(g) + __H2O(l) UV/c...
Questions

Social Studies, 30.08.2020 14:01

Mathematics, 30.08.2020 14:01

Mathematics, 30.08.2020 14:01

Chemistry, 30.08.2020 14:01

Physics, 30.08.2020 14:01

Mathematics, 30.08.2020 14:01

Biology, 30.08.2020 14:01


Social Studies, 30.08.2020 14:01


Social Studies, 30.08.2020 14:01



Spanish, 30.08.2020 14:01

Chemistry, 30.08.2020 14:01

Mathematics, 30.08.2020 14:01

Geography, 30.08.2020 14:01


Business, 30.08.2020 14:01

Mathematics, 30.08.2020 14:01