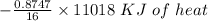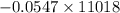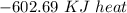Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1


Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1
You know the right answer?
Using the following equation for the combustion of octane calculate the heat associated with the for...
Questions

Physics, 25.07.2019 01:30

Mathematics, 25.07.2019 01:30



Mathematics, 25.07.2019 01:30


Physics, 25.07.2019 01:30


Mathematics, 25.07.2019 01:30








Social Studies, 25.07.2019 01:30



Mathematics, 25.07.2019 01:30

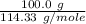
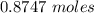
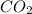 formation associates with -11018 kJ of heat, then
formation associates with -11018 kJ of heat, then