Chemistry, 28.07.2021 06:30 breannabryan1017
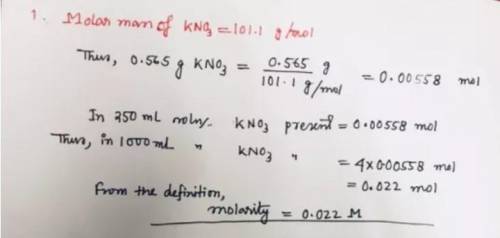
A solution is made by dissolving 0.565 g of potassium nitrate in enough water to make up 250. mL of solution. What is the molarity of this solution? Please explain and show work.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2


Chemistry, 22.06.2019 23:20
In medium-sized stars such as the sun, nuclear fusion almost always means the fusing of nuclei to form , but larger stars can produce elements as heavy as
Answers: 2

Chemistry, 23.06.2019 05:00
Match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) amount of product predicted to be produced by the given reactants theoretical yield c) reactant that can produce more of the product
Answers: 3
You know the right answer?
A solution is made by dissolving 0.565 g of potassium nitrate in enough water to make up 250. mL of...
Questions

Mathematics, 21.01.2022 18:20

Mathematics, 21.01.2022 18:20

Arts, 21.01.2022 18:20


Mathematics, 21.01.2022 18:20

Mathematics, 21.01.2022 18:20




Mathematics, 21.01.2022 18:20

Chemistry, 21.01.2022 18:20

English, 21.01.2022 18:20


Mathematics, 21.01.2022 18:20

Mathematics, 21.01.2022 18:20

English, 21.01.2022 18:20

Advanced Placement (AP), 21.01.2022 18:20


Mathematics, 21.01.2022 18:30

Mathematics, 21.01.2022 18:30