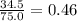Chemistry, 28.07.2021 06:30 kathleendthomas
A solution is made by mixing 34.5 g of sugar with 75.0 g of water. What is the mass percent of sugar in this solution? Please explain and show work.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:10
Which of these will change if the air in aclosed bottle is heated? abcdthe mass of the airthe composition of the airthe air pressure in the bottlethe number of air molecules in the bottle
Answers: 3

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1

Chemistry, 23.06.2019 00:00
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1

Chemistry, 23.06.2019 02:00
Why does ammonia, nh3, behave as a base when it reacts with an acid? z
Answers: 2
You know the right answer?
A solution is made by mixing 34.5 g of sugar with 75.0 g of water. What is the mass percent of sugar...
Questions


English, 09.07.2019 20:00

Social Studies, 09.07.2019 20:00

Mathematics, 09.07.2019 20:00


History, 09.07.2019 20:00


English, 09.07.2019 20:00


Mathematics, 09.07.2019 20:00

History, 09.07.2019 20:00



Mathematics, 09.07.2019 20:00

English, 09.07.2019 20:10




Mathematics, 09.07.2019 20:10

Mathematics, 09.07.2019 20:10

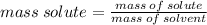 ×100
×100