
Chemistry, 28.07.2021 09:50 carolinamleal04
cân 0.6g KHS03 cho vào 500ml, nước cất, ta thu được dung dịch A. tính pH và đêm năng của dung dịch A. Biết Axit H2SO3 có hằng số phân ly Ka1=1,7.10-2, Ka2=10-7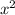

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Will give brainliest it is a lab from k12 here is the linkfor each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. type your answer here. (score for question 3: of 5 points) were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. type your answer here. (score for question 4: of 5 points) make a general statement about the reactivity of the metals in this experiment. type your answer here.
Answers: 2

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2


Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1
You know the right answer?
cân 0.6g KHS03 cho vào 500ml, nước cất, ta thu được dung dịch A. tính pH và đêm năng của dung dịch A...
Questions


English, 19.09.2019 13:50



Geography, 19.09.2019 13:50

Mathematics, 19.09.2019 13:50



Health, 19.09.2019 13:50

Spanish, 19.09.2019 13:50

Chemistry, 19.09.2019 13:50


Biology, 19.09.2019 13:50


Biology, 19.09.2019 13:50

English, 19.09.2019 13:50

History, 19.09.2019 13:50


English, 19.09.2019 13:50

Biology, 19.09.2019 13:50



