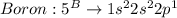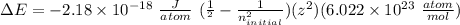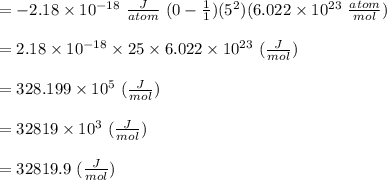Chemistry, 28.07.2021 21:00 xxheartbreakerxx11
Given the following formula for calculating the ionization energy of one-electron species such as Li2+, He+, and H, calculate the ionization energy (in J/mol) for B4+. Use scientific notation in answers (ex: 1E10, 3.20E-6)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2


You know the right answer?
Given the following formula for calculating the ionization energy of one-electron species such as Li...
Questions

Mathematics, 05.02.2021 22:00







Mathematics, 05.02.2021 22:00

Social Studies, 05.02.2021 22:00

Biology, 05.02.2021 22:00

Mathematics, 05.02.2021 22:00

Mathematics, 05.02.2021 22:00

Mathematics, 05.02.2021 22:00


Mathematics, 05.02.2021 22:00

Chemistry, 05.02.2021 22:00



Mathematics, 05.02.2021 22:00

Advanced Placement (AP), 05.02.2021 22:00

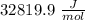 "
"