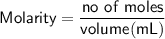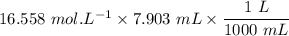Sally has constructed a concentration cell to measure Ksp for MCln. She constructs the cell by adding 2 mL of 0.05 M M(NO3)n to one compartment of the microwell plate. She then makes a solution of MCln by adding KCl to M(NO3)n. She adds 7.903 mL of the resulting mixture to a second compartment of the microwell plate. Sally knows n = +2. She has already calculated [Mn+] in the prepared MCln solution using the Nernst equation. [Mn+] = 8.279 M
Required:
How many moles of [Cl-] must be dissolved in that compartment?

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 19:00
Agood hypothesis includes which of the following? a: prediction b: data c: uncertainty d: conclusion
Answers: 1

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 23.06.2019 00:00
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1
You know the right answer?
Sally has constructed a concentration cell to measure Ksp for MCln. She constructs the cell by addin...
Questions

Mathematics, 24.02.2020 11:11

Mathematics, 24.02.2020 11:11





Mathematics, 24.02.2020 11:13

Chemistry, 24.02.2020 11:17

Mathematics, 24.02.2020 11:17

Mathematics, 24.02.2020 11:21


Geography, 24.02.2020 11:25





Mathematics, 24.02.2020 11:29

Arts, 24.02.2020 11:29

Mathematics, 24.02.2020 11:31

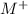 reacted with Cl⁻ to form
reacted with Cl⁻ to form 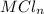 i.e. the compound formed is
i.e. the compound formed is 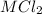 .
.![[M^+]](/tpl/images/1402/0086/bbccd.png) = 8.279 M
= 8.279 M![[Cl^-]](/tpl/images/1402/0086/0726e.png) = 2 × 8.279 M
= 2 × 8.279 M