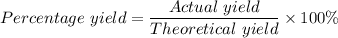Calcium oxide reacts with water in a combination reaction to produce calcium hydroxide: CaO (s) + H2O (l) → Ca(OH)2 (s) In a particular experiment, a 2.00-g sample of CaO is reacted with excess water and 2.14 g of Ca(OH)2 is recovered. What is the percent yield in this experiment? a. 107 b. 1.07 c. 2.88 d. 81.1 e. 93.3

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3


You know the right answer?
Calcium oxide reacts with water in a combination reaction to produce calcium hydroxide: CaO (s) + H2...
Questions


Mathematics, 01.06.2021 07:10


Mathematics, 01.06.2021 07:10

Social Studies, 01.06.2021 07:10

Chemistry, 01.06.2021 07:10

Mathematics, 01.06.2021 07:10

History, 01.06.2021 07:10

History, 01.06.2021 07:10

English, 01.06.2021 07:10

History, 01.06.2021 07:10

Social Studies, 01.06.2021 07:10


Mathematics, 01.06.2021 07:10

Mathematics, 01.06.2021 07:10

English, 01.06.2021 07:10

History, 01.06.2021 07:10

Mathematics, 01.06.2021 07:10

History, 01.06.2021 07:20

Chemistry, 01.06.2021 07:20