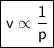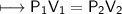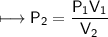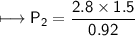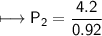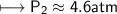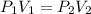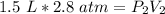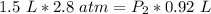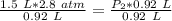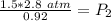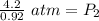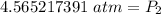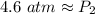Chemistry, 01.08.2021 06:00 emokid7822
At a constant temperature, a sample of gas occupies 1.5 L at a pressure of 2.8 ATM. What will be the pressure of this sample, in atmospheres, if the new volume is 0.92 L?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1

Chemistry, 23.06.2019 07:00
Choose the correct statement about licensed veterinarians in the united states. a. they must be certified by the avma. b. they can treat all nonhuman animals. c. they can can treat only animals specified on the license. d. they must choose a specialty.
Answers: 2

Chemistry, 23.06.2019 15:30
Which answer below correctly identifies the type of change and the explanation when magnesium comes into contact with hydrochloric acid
Answers: 1
You know the right answer?
At a constant temperature, a sample of gas occupies 1.5 L at a pressure of 2.8 ATM. What will be the...
Questions

Chemistry, 18.03.2021 01:50

Mathematics, 18.03.2021 01:50

Arts, 18.03.2021 01:50



Mathematics, 18.03.2021 01:50


Mathematics, 18.03.2021 01:50

Social Studies, 18.03.2021 01:50


Mathematics, 18.03.2021 01:50

English, 18.03.2021 01:50

English, 18.03.2021 01:50

Mathematics, 18.03.2021 01:50


Mathematics, 18.03.2021 01:50

Mathematics, 18.03.2021 01:50

Mathematics, 18.03.2021 01:50

Mathematics, 18.03.2021 01:50

English, 18.03.2021 01:50